Made by Chemours, sold by Nano Cats GmbH
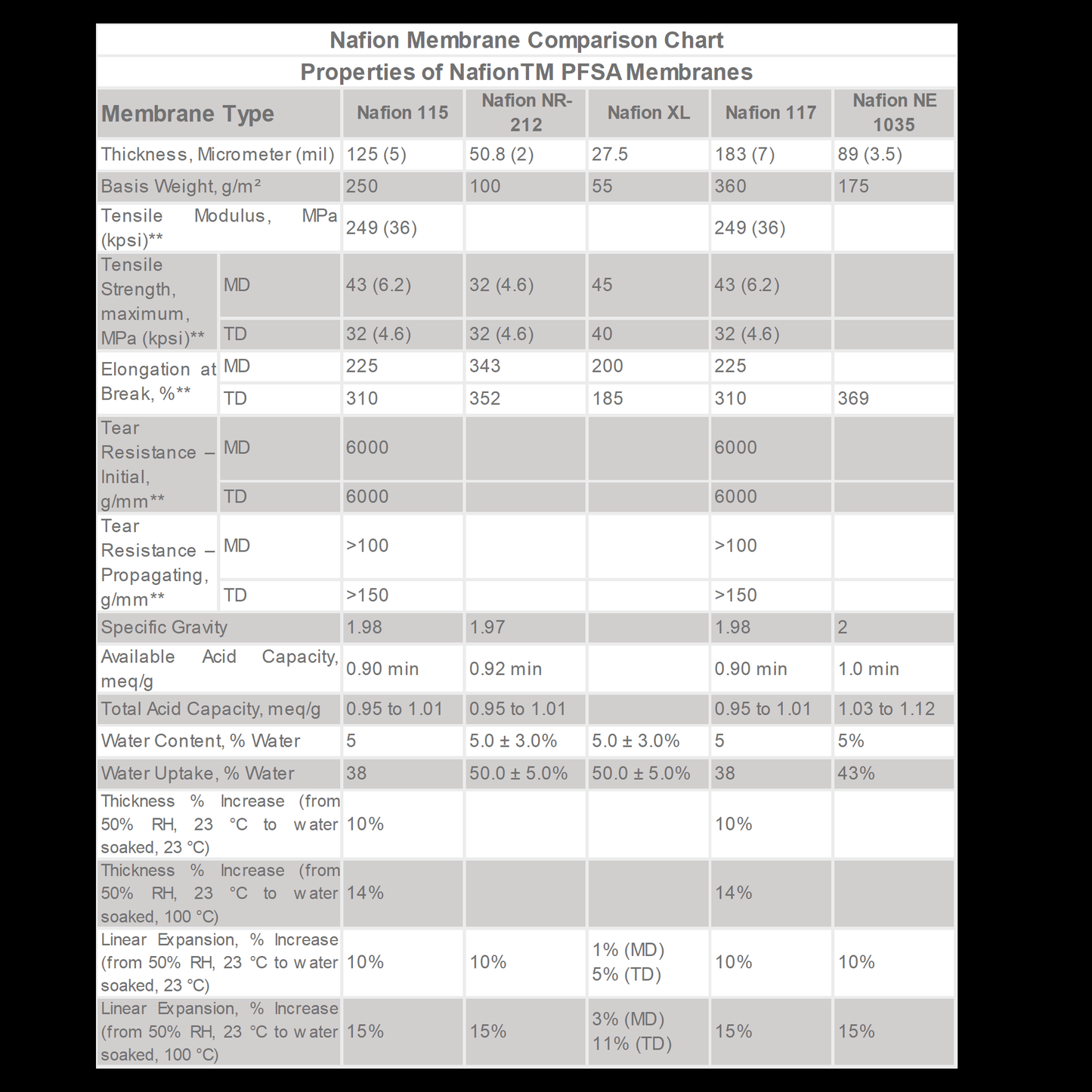
Nafion™ Membranes
Nafion™ Membranes
Couldn't load pickup availability
Introduction
Nafion™ polymer, created by Dr. Walther Grot at DuPont in the late 1960s by modifying Teflon™, was the first synthetic polymer with ionic properties, leading to the development of ionomers. Its unique ionic characteristics come from sulfonic acid groups integrated into the polymer matrix, combining the benefits of Teflon™ with ionic features, including:
Chemical Resistance: Nafion™ is highly resistant to chemical attacks, only affected by metallic alkali metals like sodium under normal conditions.
High-Temperature Stability: It can be used at temperatures up to 190°C.
Ionic Conductivity: Unlike Teflon™, Nafion™ is an effective cation exchange polymer.
Catalytic Activity: The sulfonic acid groups act as strong proton donors.
Water Permeability: Nafion™ efficiently absorbs and transfers water due to its high water-of-hydration.
Applications
Nafion™ polymer is utilized in four main areas:
Ion-Exchange Membranes: For producing chlorine gas and sodium hydroxide via electrolysis.
Gas Drying and Humidifying: In applications for anesthesia and respiratory care.
Proton Exchange Membranes: Essential for polymer electrode fuel cells and water electrolysers.
Super-Acid Catalysts: Used in fine chemical synthesis.
Physical Appearance
Initially produced in a neutralized salt form, Nafion™ polymer is thermoplastic but inactive. Upon activation, it becomes a translucent plastic, similar to Teflon™.
Once activated, it absorbs water vapor and interacts with organic gases, leading to color changes from translucent to yellow, brown, and ultimately black due to residue buildup. However, its chemical structure and core properties remain unchanged.
Under normal conditions, Nafion™ typically turns yellow within a year and brown within three to five years, yet it likely retains full functionality without performance loss. Unwanted chemical reactions can be exacerbated by light and heat, so storing Nafion™ in sealed bags away from light helps preserve its initial appearance.
Share